Nitrosamine Impurities Testing
Nitrosamine Testing : Nitrosamines Analysis
With a wealth of experience, we excel in identifying and quantifying trace impurities in a diverse range of materials and marketed products crucial to the pharmaceutical and healthcare sectors worldwide.
With complementary expertise in method development, method validation and stability testing, our analytical sciences team are the perfect partner for your project.
Contact us to discuss your nitrosamine testing project
Nitrosamine Drug Substance-Related Impurities (NDSRI) Analysis
Resolian is your trusted partner for Nitrosamine Analysis. With a wealth of experience, we excel in identifying and quantifying trace impurities in a diverse range of materials crucial to the pharmaceutical and healthcare sectors worldwide.
We have extensive experience in the analysis of a full range of nitrosamines, including both Low Molecular Weight Nitrosamines and Nitrosamine Drug Substance-Related Impurities (NDSRIs), in APIs, excipients, tablets, creams, solutions and medical devices.
GMP LC-MS/MS Development, Validation and Batch Release
We have developed highly sensitive and robust GMP LC-MS/MS methods suitable for verifying nitrosamine levels in your product for safety risk assessments and batch release
Each project team within our Nitrosamines group will be led by senior scientists highly experienced in the validation of trace methodologies in accordance with the latest ICH Q2 guidelines , ensuring the methods we develop meet your quality requirements.
Utilising these methods we will support your routine or investigational sample analysis, including GMP batch release, partnering work with you to transfer the methodologies to your QC labs or any combination of the above.
LC-MS/MS is especially suited to the analysis of nitrosamines as most are not readily analyzable using alternative detection techniques. Nitrosamines vary greatly in volatility and most importantly need to be determined at very low levels. LC-MS/MS using a triple quadrupole mass analyser operating in multiple reaction monitoring (MRM) mode, provides the best combination of sensitivity and selectivity.
We work with you to determine the best course of action for your product.
For most formulation types a limit test at an agreed Target Analytical Level (ideally set lower than the limits suggested by FDA and EMA) is generally considered to be the best approach.
Where required fully validated quantitative methods can be developed.
Maximise Confidence with HRAMS
With the increased molecular size and complexity of NDSRIs, there is an increased opportunity for isobaric impurities to induce false positives.
When a positive result is observed during targeted LC-MS/MS analysis, a more definitive assessment of the component identity may be desired.
Resolian can utilize a combination of Multi-Dimensional Liquid Chromatography, High Resolution Accurate Mass Spectrometry (HRAMS) and state-of-the-art characterization software, as required, to elucidate the structure of components detected during targeted LC-MS/MS analysis.
Our data-independent HRAMS approach enables the confident verification of the target identity as well as enabling the identification of components that could induce false positives.
Through the confirmation of component identity by HRAMS, Resolian can provide maximum confidence in the identity of positive nitrosamines results at sub-ppm levels.
Eliminate False Positives
Due to the ubiquity of nitrosation sources, false positive results have been commonplace throughout the history of generic nitrosamine testing. These false positive results have consequently lead to unnecessary financial implications. At Resolian we specialise in the efficient development of highly selective, tailored methods which differentiate between nitrosamines and structurally similar impurities that would otherwise lead to a false positive.
Where nitrosamines are detected, we will provide a package of experiments to verify the source of the observed levels; enabling the development of suitable control strategies for the levels of nitrosamine present and mitigating the risk of unnecessary product removal. An example experimental solution we employ to aid the assessment and prevention of false positives is the utilization of scavengers within the methodology.
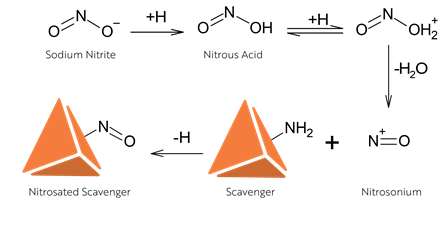
The incorporation of a viable nitrite scavenger at an appropriate stage of the methodolgy can prevent the undesired formation of nitrosamines by intercepting the nitrosation source before it can react with your sample. However, experience is the key to the successful development of a False Positive Assessment Package.
The package will depend upon the nature of the sample and your requirements, as such we encourage a collaborative relationship to efficiently arrive at the desired outcome.
Contact us to discover bespoke solutions perfectly tailored to your specific requirement

The next chapter in Nitrosamine Testing
We have seen a significant increase in requests related to API related nitrosamines.
