Document Writing and Publishing
Our dedicated Document Writing and Publishing Team support the production of documents in DDS.
We are experienced in providing high quality reports to our own house style, with the additional flexibility to customise to Sponsor requirements (when requested), no matter the size of document.
Writing reports
Within the Document Writing and Publishing Team, we have a variety of different scientific expertise, covering the range of department areas within Drug Development Solutions. With backgrounds in report writing, regulated project management and project co-ordination for both LC-MS and Immunoassay departments.
By using our library of study specific templates and house style we are able to write high quality reports, adapting them when required to our Sponsor’s needs. We work closely with the Project Manager within DDS or the external Sponsor, to ensure that the requirements of each project are met.
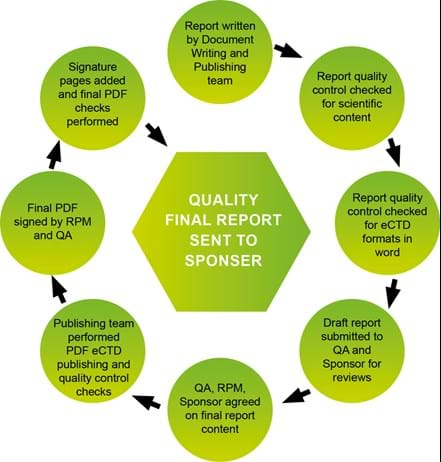
We have a multilevel quality control check process for report writing and report eCTD document level publishing work, ensuring the quality of our documents.
Publishing services
Within the Document Writing and Publishing Team, we provide Document level eCTD publishing using Adobe Acrobat software. Following eCTD guidelines, we work towards the DDS eCTD publishing policy however we can customise to Sponsor requirements (on request) . With various quality control checks, including use of the publishing software, we ensure the quality of our products.
