CASE STUDY – ANTI-AAV ANTIBODIES
We are experienced with a wide range of different proteins and therapeutics.
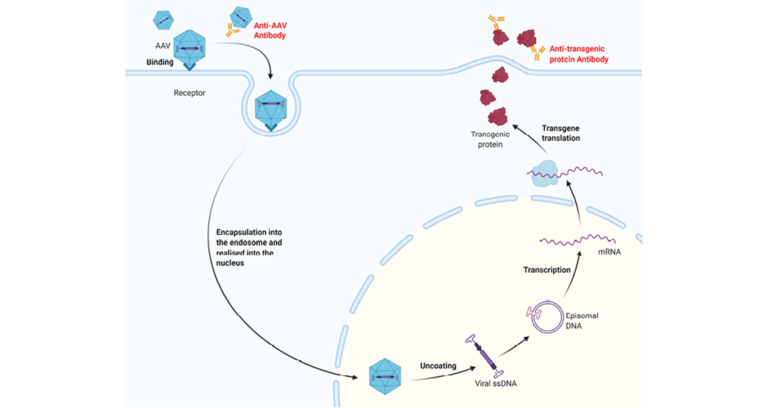
Adeno-associated viruses (AAV) are small viruses that infect humans and some other primate species. The AAV is transformed from a naturally occurring virus into a delivery mechanism for gene therapy.
Our customer needed to develop and validate an Immunogenicity assay to measure the presence of preexisting antibodies, due to previous naturally occurring AAV infections, and antibodies generated to the AAV delivery vehicle.
Challenges
- Challenges labelling AAV for bridging format
- Negative inhibition observed in some individuals suggesting hook effect in the confirmatory assay
- High prevalence of anti-AAV in general population therefore difficult to produce a true NC
- No quantifiable PC available, only positive samples
- Guidance is lacking for detection of anti-AAV antibodies: but FDA/EMA generally used
Goal
To develop and validate an immunogenicity assay to determine the presence of specific anti-AAV antibodies pre- and post- gene therapy.
Tiered approach to sample analysis
Immunogenicity assay approach for detection of anti-AAV antibodies.
Our solution
In the absence of detailed regulatory guidelines for the detection of anti-AAV antibodies the Immunogenicity Centre of Excellence used in-house bioconjugation and strategies to develop a bridging assay format as well as cope with the high prevalence of anti-AAV in the general population which meant production of a true negative control serum pool was challenging.
The identification of a true Negative Control (NC) pool was an essential reagent for the successful validation of the immunogenicity assay to detect specific anti-AAV antibodies in human, pre- and post- gene therapy.
Our process
On site LC-MS technology confirmed successful bio-conjugation of AAV with both MSD GOLD™ SULFO-TAG NHS-Ester C and biotin, and suitability for use within the bridging assay.
This enabled characterization and control of these critical reagents.


The Immunogenicity Centre of Excellence successfully identified a suitable NC, develop, optimise the MRD and assay to reduce confirmatory hook effect and validate a fit-for-purpose assay and liaise with the Native Antigen Group to produce a quantifiable Positive Control (PC).
Results
- Anti-AAV capsid immunogenicity assay developed/validated
- Transgenic protein PK and immunogenicity assays developed/validated
Purification of Positive Control
- Proof of concept tested in house using HiTrap resin
- Outsourced to member of LGC group:
Native Antigen Company
- Quantifiable PC available for validation
- High individual responders also included to monitor assay range
Validation
- The validated assay (screening, confirmatory and titre) will support sample analysis throughout the method lifecycle
- Correct tools and robust assay lead to successful validation
- Assay fit-for-purpose
- FDA/EMA immunogenicity guidance “followed in spirit”

