Discovery, Preclinical & Tissue Bioanalysis
Non-GLP and Discovery Bioanalysis at Drug Development Solutions
We understand the cost and time pressures on your discovery and lead identification projects. To meet these demands, we have developed a rapid, standardised, efficient approach to discovery bioanalysis that helps you complete your projects with confidence, on time and on budget.
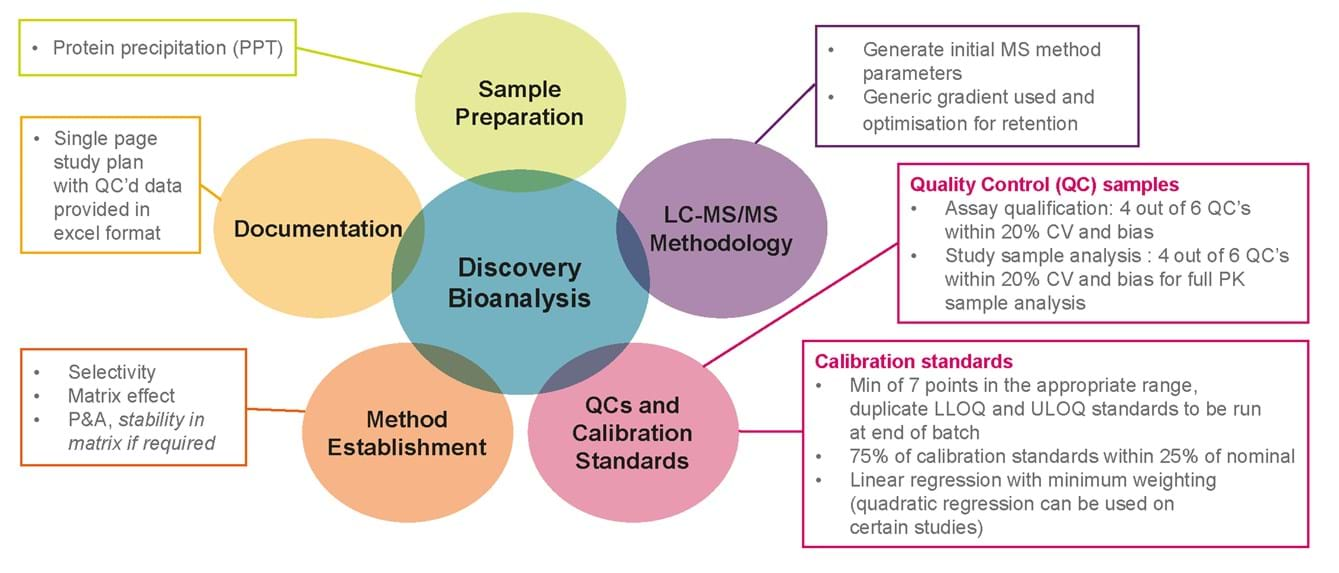
Our key steps to discovery bioanalysis
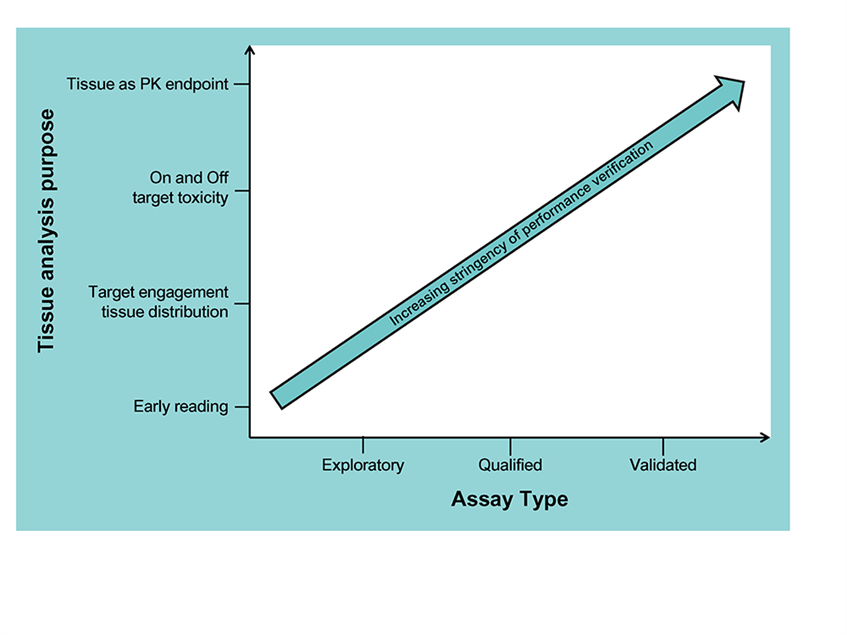
Tissue Bioanalysis
Our bioanalysis team has over 10 years’ experience and a proven track record of analysing a wide variety of drugs (including small molecules to proteins and endogenous molecules) from a variety of tissues and species supporting both regulated and non-regulated studies.
Typically we can achieve quantitation in the pg/mL to ng/mL range in our tissue LC-MS/MS assays. Matrix matching as close as possible to matrix and species is preferred but in some instances surrogate matrices made be used for tissues with limited supply.
Preclinical studies and beyond
Once your molecule is ready to progress to pre-clinical studies, we can tailor our approach to meet your needs. Depending on the regulatory application of data generated, we are able to undergo a full method development activity and validation to EMA guidelines. This ensures we can support the progression of a chosen candidate molecule from discovery through to regulated preclinical studies and beyond.
 Integrated LC-MS bioanalysis solutions
Integrated LC-MS bioanalysis solutions
Our Bioanalysis group has a unique 50+ year history that has fuelled our growth into a leading specialist bioanalytical provider. Based in Cambridge (Fordham), our LC-MS/MS team consists of dedicated scientists recognised for leadership in science and technology. The Drug Development Solutions approach provides an integrated solution of experts throughout the drug development pipeline with a single point of contact facilitating superior levels of accountability, efficiency and clarity of communication.
Our integrated services include:
- Fast method development for discovery compounds
- Method development and validation for GLP TK studies and GCP clinical trials
- Fast turnaround for Phase I Clinical Studies
- Pharmacokinetic data analysis using validated software systems (e.g. WinNonlin)
- Logistical support for clinical sample management including sample collection kit production production and supply of sampling kits, labels, investigator manual and shipping
- Data management using LIMS or specialist software
