A method to measure anti-vaccine antibodies
CASE STUDY - Antibody Assay

The need
An antibody assay was required to detect and quantify IgGs produced against a vaccine. This was required to measure the efficacy and reliability of the vaccine.
Challenges
- Quantification of antibodies against four different antigens; each requiring a full analytical range of quantification
- Titrated samples being analysed on four different antigen coated plates at the same time, without losing accuracy
- Finding incurred samples and a chosen reference serum that will represent the correct population
- Lack of guidance
There is a lack of guidelines for the bioanalytical community regarding developing and validating vaccine antibody assays. At Drug Development Solutions (DDS) the Immunogenicity Centre of Excellence team followed recommendations in a recent AAPS paper (2021) to validate an antibody assay to measure anti-vaccine antibodies (AVAs).
To measure AVAs, samples were titred down and concentrations calculated against a standard curve.
The method was validated using the following:
- 11 non-zero calibrators
- HQC, MQC and LQC
- Precision and accuracy
- Parallelism and linearity
- Limit of detection
- Stability assessments
- Manual vs automation comparability
Our Cambridge site is equipped with automated lab equipment, allowing for an increase in sample throughput, assay precision and accuracy, whilst reducing the labour-intensive assay.
The validated method detects antibodies against multiple antigens which the vaccine is composed off, with a primary endpoint as antibody concentrations it supports analysis in clinical trial samples for the sponsor.
This achieved an assay that was aligned with AAPS recommendations to reproducibly quantify the AVAs in samples.
Assay Design
The assay chosen uses a standard colourimetric ELISA method.
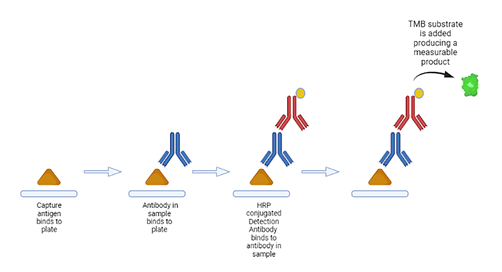
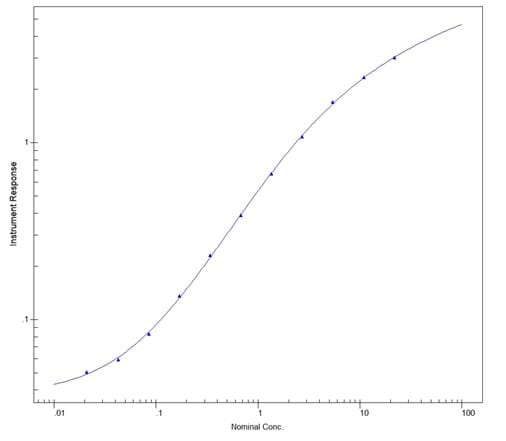
A reference serum produced from incurred samples is diluted down in assay buffer to produce a calibration curve.
Automation
Automation was introduced to increase throughput of samples, improve assay precision, and achieve reliable and accurate results.
- Hamilton Microlab STAR
- Integra Viaflo Electronic Pipette
Results
- Optimisation of the standard curve and initial dilutions of the samples enabled all four AVA concentrations to be back calculated within the analytical range
- The method validated detects antibodies against multiple vaccine/pathogen antigens to support sample analysis
- Using a fit for purpose approach, starting dilutions were optimised to allow for results to meet reporting criteria; mean for each sample concentration calculated from at least three parallel back-calculated concentrations
- An assay was achieved that aligns with AAPS recommendations to reproducibly quantifies the AVAs in samples
- Limit of detection for all four antigen concentrations was below 80 ng/mL
- Hamilton robot was used to dilute down samples, and the reference serum (to produce the standard curve), and pipette all samples into plates for analysis. Integra Viaflo was used to stamp reagents onto plates and duplicate samples Inter assay precision seen <10% for all QCs
Download the case study PDF
Case Study A Method To Measure Anti Vaccine Antibodies
1.1MB pdf
Want to learn more about Drug Development Solutions?
Our team are ready to discuss your project and answer any questions you have
